Flexible and Reliable with All the Features CRO Needs
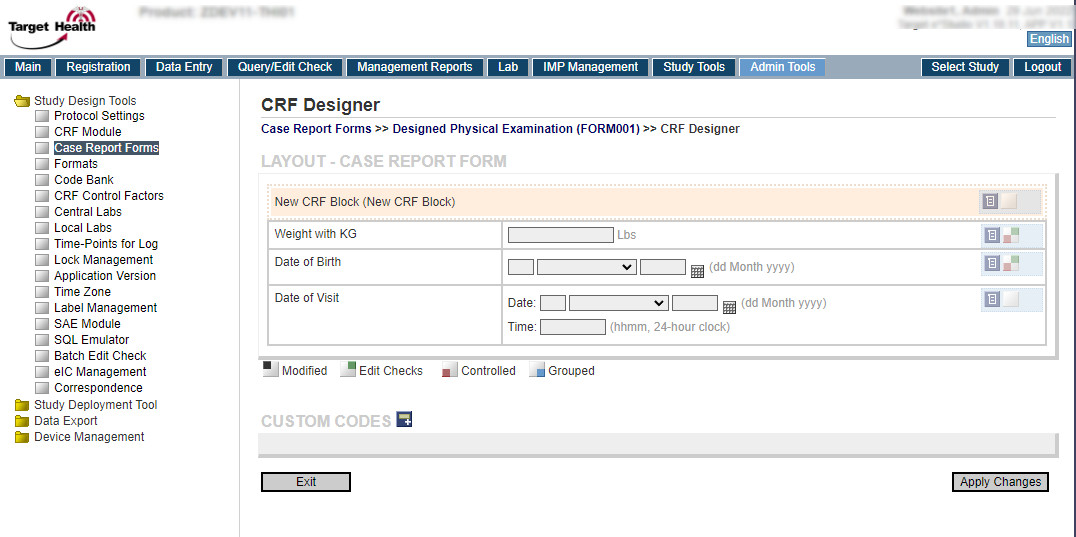
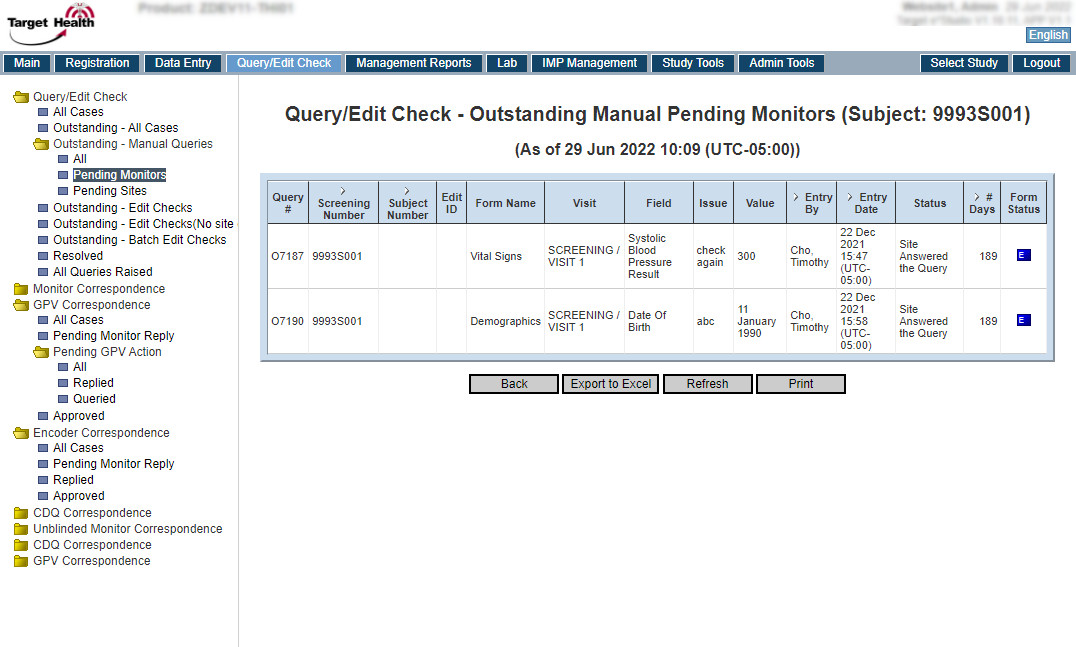
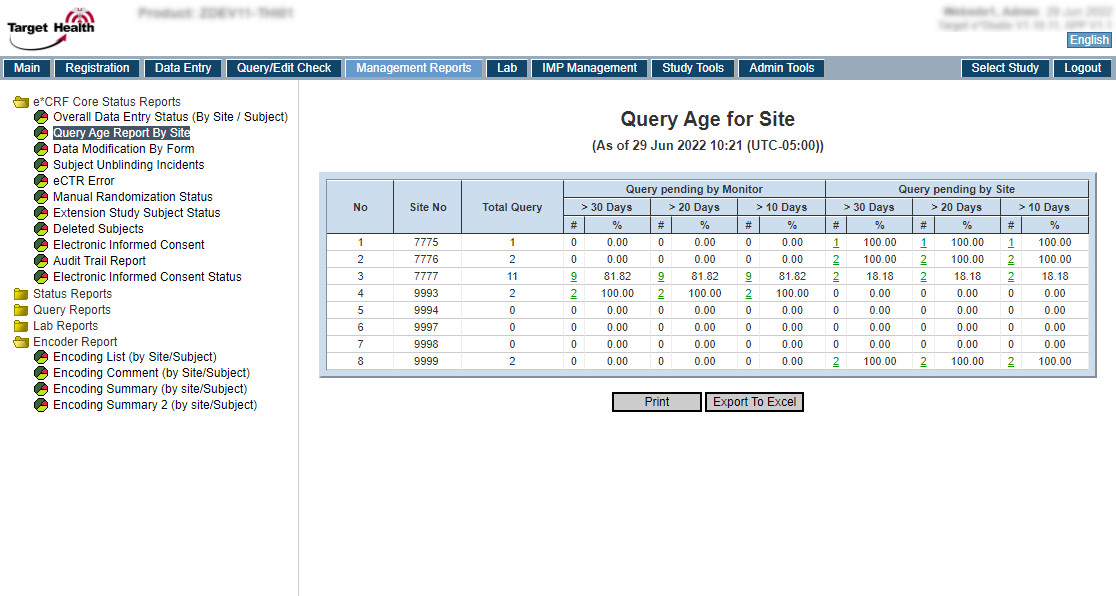
| Features | Target e*CRF + Target e*Studio |
|---|---|
| Accessibility and visibility by Role | |
| Accessibility and visibility by User | |
| Subject Multiple Locks | |
| Communication between Roles | |
| Data Exporting to Extension Study | |
| Central and Local Lab | |
| Notification from the system | |
| Protocol Deviation | |
| Medical Coding | |
| SAE Handling (Target e*Pharmacovigilance) | |
| Remote SVD (File upload/download) | |
| Randomization | |
| Unblinding | |
| IMP | |
| ePRO | |
| eIC (Target e*ICF™) | |
| Monitoring Report | |
| Data transfer to third party system | |
| Data Export (CSV, Excel, Text and SAS Dataset) | |
| Average Implementation Time | < 3 Months |
| Average Required User Training | 1 - 2 Hours |